The falsification of clinical trial data has been serious
in China. For example, in May of 2015, the
State Food and Drug Administration of China (“SFDA”) requested pharmaceutical enterprises
to do a voluntary self-inspection of their clinical data regarding the drugs to
be authorized. Those who do not would
face a more severe punishment. One month
later, 20% of 1622 applications were withdrawn.
It seems an open secret that every link of clinical trial is problematic
and it is not surprising at all that clinical trial data could be falsified in
a serious way.
There are several reasons for the falsifications.
First
of all, clinical study organizations are extremely insufficient. By statics of 2012, there were 6000 drug
research and development enterprises in China, but there were only 379 clinical
study organizations. In the year of
2012, there were as many as 704 clinical research projects being launched. Take into consideration the projects that
were extended into 2012 from the previous years, every clinical study
organization was over-burdened.
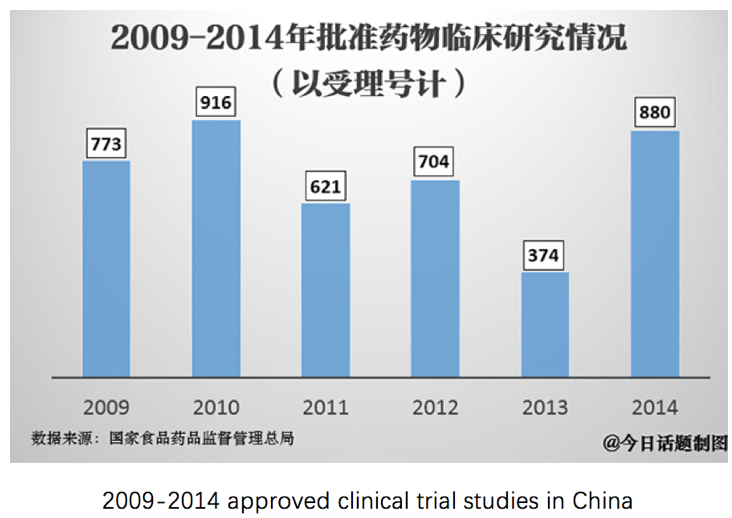
2009-2014 approved clinical trial
studies in China
Secondly,
clinical study organizations are short of experienced doctors to do clinical
studies. In China, clinical study
organizations are nothing but well-established hospital; the persons that are
responsible for clinical trials are famous doctors. The famous doctors could be too busy to do studies
nicely. They would let the doctors that
are less busy handle the studies, which are usually young and inexperienced.
Thirdly,
hospitals usually try to maximize their financial gains from clinical studies
regardless the study quality. The fund
that a drug manufacturer pays to a hospital for clinical trial could be
hair-cut by hospital and the concerned clinic(s). Only a portion of the fund could be then used
for clinical trials. Some hospitals try
to maximize their earnings by overpromising drug manufacturers, and some
hospitals even guarantee that the experiments would 100% meet the targeted indicators.
CROs
are not helpful in curbing the falsifications.
Some CROs even charge money on a contingent basis – no success in a
clinical trial, no fee. Some drug
manufacturers clearly know that some CROs are cooking data, but keep a blind
eye to it.
To
crack down on data cooking, the Supreme People’s Court (“SPC”) and Supreme
People’s Prosecutors (“SPP”) issued the Interpretations
in Several Issues on the Application of Law in Criminal Cases Regarding the
Falsifications of Application Materials for the Registration of Pharmaceuticals
and Medical Devices on August 14, 2017, and the judicial interpretation has
been in effect from September 1.
The
judicial interpretation is trying to make Article 229 of China’s Criminal Law
more criminally deterring in falsification in the life science industry, which
reads: “if a member of an intermediary organization, whose duty is to make
capital assessments, verification or validation, to do accounting or auditing,
or to provide legal services, etc., deliberately provides false documentary
evidence, and if the circumstances are serious, he or she shall be sentenced to
fixed-term imprisonment of not more than five years or criminal detention and
shall also be fined.” As such, clinical
study organizations (i.e., laboratories and healthcare institutions) and CROs
are all subject to this Article 229.
According
to the judicial interpretation, serious circumstances which would cause severe
punishments include using falsified study-related drugs, failure to report
severe adverse events, destroying original study data, falsifying study data,
having a history of submitting falsified documents in connection with the
registration of drugs or medical devices, or other circumstances that are
deemed serious. In furtherance,
soliciting or accepting “money or property” during the course of the aforesaid
misconduct can be subject to five to ten years of imprisonment and criminal
fines.
Employees
of companies applying for drug registration (i.e., sponsors) are subject to
criminal liabilities if they knowingly use organizations that do not have the
appropriate qualifications to handle clinical trials or non-clinical research,
or “the amount of payment is obviously different from usual cost.”
Those
who are interested in knowing more the subject matter issues please contact Henry.Chen@dentons.cn. Henry Chen is licensed to practice law in
China and the New York of the USA. Henry
Chen is a member of China delegation to negotiate and draft ISO 19600
Compliance Management System – Guidelines and the Vice Director of the Working
Group of China domestic standard on compliance management system. Henry is the author of the book: Risk Management on Commercial Bribery in China. Extension reading in Chinese 《合规保障、精准营销 | 中国合规网与康柏汉森达成战略合作在生命科学领域共同推广、实施合规管理》






